How is bromine produced commercially?
The industrial production of bromine involves the direct reaction of chlorine with brine rich in bromine ions. The process is fast, simple and relatively economical.
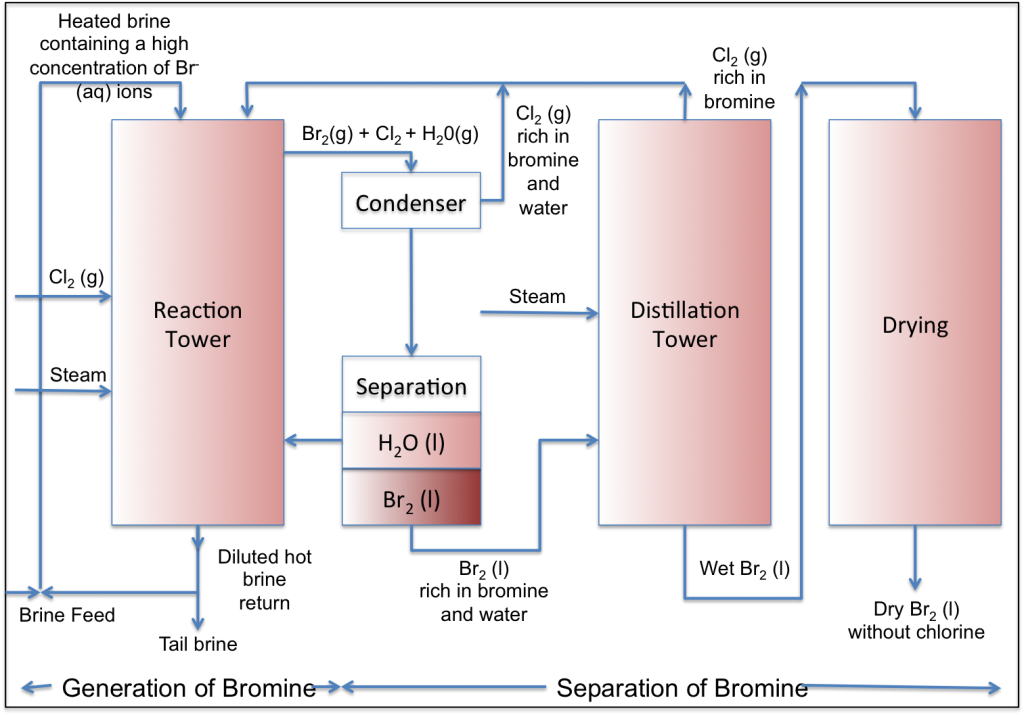
Bromine production is based on the direct feed of brine rich in bromine ions, chlorine, and steam into the reaction tower. The following block flow diagram illustrates the concept of a bromine production process.
Bromine-generating Step
Hot brine rich in bromine ion is input to the reaction tower from above, while chlorine gas and water are introduced from the bottom of the reaction tower. In the reaction tower an oxidation-reduction reaction takes place, generating bromine:
2Br-(aq) + Cl2(g) → Br2(g) + 2Cl-(aq)
It is necessary to heat the system to prevent the bromine from remaining in solution. Heating is provided by steam, which actually serves two purposes:
- Heating of the reaction tower above 100°C (100°C –120°C).
- Removal of the bromine from the solution (the steam carries the bromine vapors with it).
The gas mixture containing the bromine vapors, residual chlorine and steam rises to the top of the tower, while the liquid brine accumulates at the bottom of the tower. The tower is packed with suitable filling materials (rings or disks made from resistant materials) to increase the contact area and the reaction time between the gases and the solution.
Bromine Separation Steps
A mixture of hot gases containing bromine, chlorine and water vapor leaves the top of the tower. This mixture undergoes a number of work-up steps.
1. Condensation
The first step is to cool the gas mixture. The hot gas mixture arrives in the condenser, which has a temperature at which bromine, but not chlorine, condenses. At the temperature conditions in the condenser, the chlorine gas is separated from the liquid and after leaving the bromine and water-rich condenser it is returned to the reaction tower. The liquid phase containing chlorine and water-containing bromine is transferred t0 a separator.
2. Separation
Two layers are formed in the separator. The heavy, lower layer is the bromine. The lighter, upper layer is the aqueous layer. The aqueous layer contains bromine and chlorine, which are slightly soluble in water. After separation, this layer is recycled to the reaction tower. The bromine layer, which contains chlorine and water as impurities, is further purified as necessary.
3. Purification and Drying
Bromine obtained after the separation step is not completely pure and contains chlorine and water. The chlorine and most of the water are separated by distillation and recycled to the reaction tower. Residual water is removed by a drying process, such as by treatment of wet bromine with concentrated sulfuric acid.
Process Improvements
Today, bromine manufacturing process has evolved into the use of vacuum recovery of bromine, and the steps for generating and distilling bromine have merged into one unit operation.